Physiological Modes of Action across Species and Toxicants: The Key to Predictive Ecotoxicology
Ashauer R & Jager T (2018): Physiological Modes of Action across Species and Toxicants: The Key to Predictive Ecotoxicology. Environmental Sciences: Processes & Impacts. (link to paper, open access)
As ecotoxicologists we strive for a better understanding of how chemicals affect our environment. Humanity needs tools to identify those combinations of man-made chemicals and organisms most likely to cause problems. In other words: which of the millions of species are at risk from pollution? And which of the tens of thousands of chemicals contribute most to the risk? We identified our poor knowledge on physiological modes of action (how a chemical affects the energy allocation in an organism), and how they vary across species and toxicants, as a major knowledge gap. We also find that the key to predictive ecotoxicology is the systematic, rigorous characterization of physiological modes of action because that will enable more powerful in vitro to in vivo toxicity extrapolation and in silico ecotoxicology. In the near future, we expect a step change in our ability to study physiological modes of action by improved, and partially automated, experimental methods. Once we have populated the matrix of species and toxicants with sufficient physiological mode of action data we can look for patterns, and from those patterns infer general rules, theory and models.
General introduction to TKTD modelling

Toxicokinetic-toxicodynamic (TK-TD) models simulate the processes that lead to toxicity at the level of organisms over time. These dynamic simulation models quantify toxicity,
but more importantly they also provide a conceptual framework to better understand the causes for variability in different species' sensitivity to the same compound as well as causes for
different toxicity of different compounds to the same species. Thus TK-TD models bring advantages for very diverse ecotoxicological questions as they can address two major challenges: the large
number of species that are potentially affected and the large number of chemicals of concern. The first important benefit of TK-TD models is the role that they can play to formalize established
knowledge about toxicity of compounds, sensitivity of organisms, organism recovery times and carry-over toxicity. The second important aspect of TK-TD models is their ability to simulate temporal
aspects of toxicity which makes them excellent extrapolation tools for risk assessment of fluctuating or pulsed exposures to pollutants.
(link at journal) or (download here)
Death Dilemma and Organism Recovery in Ecotoxicology
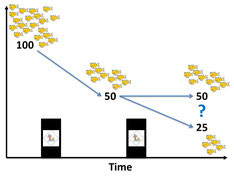
Why do some individuals survive after exposure to chemicals while others die? Either, the tolerance threshold is distributed among the individuals in a population, and its exceedance leads to certain death, or all individuals share the same threshold above which death occurs stochastically. The previously published General Unified Threshold model of Survival (GUTS) established a mathematical relationship between the two assumptions. According to this model stochastic death would result in systematically faster compensation and damage repair mechanisms than individual tolerance. Thus, we face a circular conclusion dilemma because inference about the death mechanism is inherently linked to the speed of damage recovery. We provide empirical evidence that the stochastic death model consistently infers much faster toxicodynamic recovery than the individual tolerance model. Survival data can be explained by either, slower damage recovery and a wider individual tolerance distribution, or faster damage recovery paired with a narrow tolerance distribution. The toxicodynamic model parameters exhibited meaningful patterns in chemical space, which is why we suggest toxicodynamic model parameters as novel phenotypic anchors for in vitro to in vivo toxicity extrapolation. GUTS appears to be a promising refinement of traditional survival curve analysis and dose response models. (Published in Environmental Science & Technology, open access)

We found that toxicodynamic parameters cluster according to chemical class. Clustering of toxicodynamic parameters according to chemical MOA is more pronounced for GUTS-IT (panels C, D) than for GUTS-SD (panels A, B). (A) Parameters z and kr in GUTS-SD, (B) Parameters kr and z in GUTS-SD, (C) Parameters α and β in GUTS-IT, (D) Parameters kr and α in GUTS-IT. Green squares: baseline toxicity, blue hexagons: uncoupling of oxidative phosphorylation, orange triangles: AChE inhibition (carbamate), red circles: AChE inhibition (organophosphates), purple diamonds: reactive toxicity.
This could have several applications: First, analyzing survival data in combination with toxicokinetics could indicate the mode of action. Second, it might be possible to predict the toxicity of
untested chemicals of a known mode of action by reading GUTS parameters from this figure and combining them with toxicokinetics to calculate a toxicity estimate.
Third, we suggest toxicodynamic parameters as novel phenotypic anchors for in vitro to in vivo toxicity extrapolation. Toxicity extrapolation from in vitro to in vivo systems should aim at
predicting TK-TD model parameters on the organism level as they have a biological interpretation and appear to reflect the biochemical mechanisms of toxicity. For the same reasons TK-TD
parameters may be potentially powerful end points for novel Quantitative Structure Activity Relationships. (read more: Environmental Science & Technology, open access)
TKTD models and Adverse Outcome Pathways
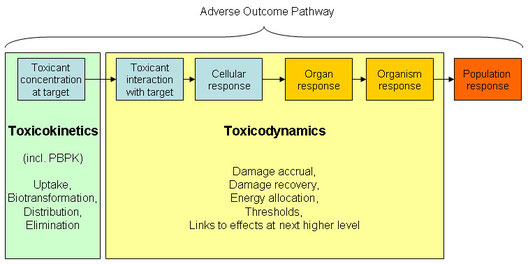
This scheme illustrates the relation of different conceptual models and terms. Toxicokinetic-toxicodynamic models are quantitative models for those entities and processes that make up the Adverse Outcome Pathways.
Adverse Outcome Pathways were proposed as a conceptual framework for risk assessment of chemicals (Ankley G, et al. (2010): Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry 29(3), 730-741). (link to journal)
Application of TKTD model in pesticide risk assessment

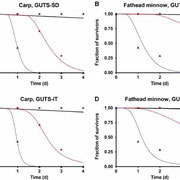
"A method to predict and understand fish survival under dynamic chemical stress using standard ecotoxicity data." Environmental Toxicology & Chemistry (link at ET&C, open access)
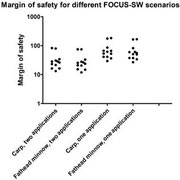
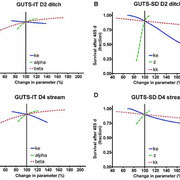
We present a method to predict fish survival under exposure to fluctuating concentrations and repeated pulses of a chemical stressor. The method is based on toxicokinetic-toxicodynamic modeling using the general unified threshold model of survival (GUTS) and calibrated using raw data from standard fish acute toxicity tests. The model was validated by predicting fry survival in a fish early life stage test. Application of the model was demonstrated by using Forum for Co-ordination of Pesticide Fate Models and Their Use surface water (FOCUS-SW) exposure patterns as model input and predicting the survival of fish over 485 d. Exposure patterns were also multiplied by factors of five and 10 to achieve higher exposure concentrations for fish survival predictions. Furthermore, the authors quantified how far the exposure profiles were below the onset of mortality by finding the corresponding exposure multiplication factor for each scenario. The authors calculated organism recovery times as additional characteristic of toxicity as well as number of peaks, interval length between peaks, and mean duration as additional characteristics of the exposure pattern. The authors also calculated which of the exposure patterns had the smallest and largest inherent potential toxicity. Sensitivity of the model to parameter changes depends on the exposure pattern and differs between GUTS individual tolerance and GUTS stochastic death. Possible uses of the additional information gained from modeling to inform risk assessment are discussed.
Calibration, predictive power and do we need internal concentrations?
Using the General Unified Threshold model of Survival this study systematically investigates how well different ways of using the model perform when it comes to predicting survival: "Toxicokinetic-toxicodynamic modelling of survival of Gammarus pulex in multiple pulse exposures to propiconazole: model assumptions, calibration data requirements and predictive power." Ecotoxicology (link to paper, free access)
Toxicokinetic-toxicodynamic (TKTD) models quantify the time-course of internal concentration, which is defined by uptake, elimination and biotransformation (TK), and the processes which lead to the toxic effects (TD). TKTD models show potential in predicting pesticide effects in fluctuating concentrations, but the data requirements and validity of underlying model assumptions are not known. We calibrated TKTD models to predict survival of Gammarus pulex in propiconazole exposure and investigated the data requirements. In order to assess the need of TK in survival models, we included or excluded simulated internal concentrations based on pre-calibrated TK. Adding TK did not improve goodness of fits. Moreover, different types of calibration data could be used to model survival, which might affect model parameterization. We used two types of data for calibration: acute toxicity (standard LC50, 4 d) or pulsed toxicity data (total length 10 d). The calibration data set influenced how well the survival in the other exposure scenario was predicted (acute to pulsed scenario or vice versa). We also tested two contrasting assumptions in ecotoxicology: stochastic death and individual tolerance distribution. Neither assumption fitted to data better than the other. We observed in 10-d toxicity experiments that pulsed treatments killed more organisms than treatments with constant concentration. All treatments received the same dose, i.e. the time-weighted average concentration was equal. We studied mode of toxic action of propiconazole and it likely acts as a baseline toxicant in G. pulex during 10-days of exposure for the endpoint survival.
General Unified Threshold model of Survival (GUTS)
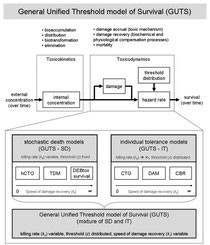
Jager T, Albert C, Preuss TG, Ashauer R (2011): General unified threshold model of survival - a toxicokinetic-toxicodynamic framework for ecotoxicology. Environmental Science and Technology, 45(7), 2529-2540. (link at journal) or (download here)
Toxicokinetic-toxicodynamic models (TKTD models) simulate the time-course of processes leading to toxic effects on organisms. Even for an apparently simple endpoint as survival, a large number of very different TKTD approaches exist. These differ in their underlying hypotheses and assumptions, although often the assumptions are not explicitly stated. Thus, our first objective was to illuminate the underlying assumptions (individual tolerance or stochastic death, speed of toxicodynamic damage recovery, threshold distribution) of various existing modeling approaches for survival and show how they relate to each other (e.g., critical body residue, critical target occupation, damage assessment, DEBtox survival, threshold damage). Our second objective was to develop a general unified threshold model for survival (GUTS), from which a large range of existing models can be derived as special cases. Specific assumptions to arrive at these special cases are made and explained. Finally, we illustrate how special cases of GUTS can be fitted to survival data. We envision that GUTS will help increase the application of TKTD models in ecotoxicological research as well as environmental risk assessment of chemicals. It unifies a wide range of previously unrelated approaches, clarifies their underlying assumptions, and facilitates further improvement in the modeling of survival under chemical stress.
TKTD models for sublethal endpoints
Ashauer R, Agatz A, Albert C, Ducrot V, Galic N, Hendriks J, Jager T, Kretschmann A, O'Connor I, Rubach MN, Nyman A-M, Schmitt W, Stadnicka J, van den Brink PJ, Preuss TG: (2011): Toxicokinetic-toxicodynamic modeling of quantal and graded sublethal endpoints: A brief discussion of concepts. Environ Toxicol Chem 30:2519-2524. (link to journal here or download from here)
We report on the advantages and problems of using toxicokinetic-toxicodynamic (TKTD) models for the analysis, understanding, and simulation of sublethal effects. Only a few toxicodynamic approaches for sublethal effects are available. These differ in their effect mechanism and emphasis on linkages between endpoints. We discuss how the distinction between quantal and graded endpoints and the type of linkage between endpoints can guide model design and selection. Strengths and limitations of two main approaches and possible ways forward are outlined.
Design of experiments for TKTD model parameterisation
Albert C, Ashauer R, Künsch HR, Reichert P (2012): Bayesian Experimental Design for a Toxicokinetic-Toxicodynamic Model. Journal of Statistical Planning and Inference, 142, 263-275. (link at journal) or (download here) Here are some indications on how to design the experiments:
- Use multiple pulses in sequence if you want to learn about model structure (e.g. the need for a damage variable) and if you want to test for carry-over toxicity.
- Use one (or few) intense pulses in at least one treatment
- Use longer, lower constant exposure in another treatment
Of course I recommend to read that paper and some others to learn about the details...